Scientific Leaders of the project: Javier García, José Luis Pedraz, Simó Schwartz & Ramón Mangues.
Coordinator of the project: Ibane Abasolo & Javier García
One of the causes that increase the time to market of nanotherapeuticsis its preclinical validation. The standard protocols for more traditional drugs are not applicable for nanomedicines. NANBIOSISoffers a preclinical study plan tailored to meet the demands of each nanomedicine product, developed in collaboration with the user and starting from a set of basic analytical tests to more sophisticated experiments.
We offer a complete In vitro Characterization. e.g., immunology, cytotoxicity, hematology, oxidative stress, etc, and more adapted to the different products by using the most sophisticated equipment and taking advantage of the expertise of scientist internationally recognized in the matter.
Sodiumcolistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. M. Pastor. M. Moreno-Sastre, A. Esquisabel, E. Sans, M. Viñas, D.lBachiller, V. J. Asensio, Á. Del Pozo, E. Gainza, J. L. Pedraz. Int. J. of Pharm. 477 (2014) 485-494.
Intrapericardial Delivery of Cardiosphere-Derived Cells: An Immunological Study in a Clinically Relevant Large Animal Model. Blázquez R, Sánchez-Margallo FM, Crisóstomo V, Báez C, Maestre J, Álvarez V, Casado JG. PLoS One. 2016 Feb 11;11(2):e0149001. doi: 10.1371/journal.pone.0149001. eCollection 2016.
Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Front Immunol. 2014 Nov 4;5:556. doi: 10.3389/fimmu.2014.00556. eCollection 2014.
Services involved:
| Sterility | Gel-Clot LAL Assay | U20 |
| Detection of Microbial Contamination | U20 | |
| Detection of Bacterial Contamination | U20 | |
| Detection of Mycoplasma Contamination | U20 | |
| Immunology | Analysis of Hemolytic Properties of Nanoparticles | U20 |
| Analysis of Platelet Aggregation by Cell Counting | U20 | |
| Analysis of Platelet Aggregation by Light Transmission Aggregometry | U20 | |
| Analysis of Nanoparticle Interaction with Plasma Proteins by 2D PAGE | U20 | |
| Leukocyte Pro life ration Assay | U14 | |
| Detection of Nitric Oxide Production by RAW 264. 7 MacrophageCell Line | U18 | |
| ChemotaxisAssay | U18 | |
| PhagocytosisAssay | U18 | |
| Analysis of Cytokines, Chemokines and Interferons (by Elisa or Multiplex) IL-8, IL-1b, TNF-α, IFN- ϒ and many others | U14 | |
| Determination of cytokine concentration by flow cytometry | U14 | |
| Measurement of Nanoparticle Effects on Cytotoxic Activity of NK Cells by Label-Free RT-CES System | U14 (consult) | |
| In vitro Induction of Leukocyte Procoagulant Activity by Nanoparticles | U20 (consult) | |
| Oxidative Stress | Hep G2 Hepatocyte Glutathione Assay | U20 |
| Hep G2 Hepatocyte Lipid Peroxidation Assay | U20 (consult) | |
| HepatocytePrimary ROS Assay | U20 (consult) | |
| Cytotoxicity (necrosis) | Cytotoxicity (necrosis) Assay (MTT and LDH Release): LLC-PK1 Kidney, Hep G2 Hepatocarcinoma, etc | U10, U18 & U20 |
| Cytotoxicity (apoptosis) | Cytotoxicity (apoptosis) (Caspase 3 Activation) LLC-PK1 Kidney; Hep G2 Hepatocarcinoma ; etc | U10 U18 |
| Cytotoxicity (apoptosis) (Caspase 3/7 Activation) Hep G2 Hepatocarcinoma ; etc | U10 & U18 | |
| Autophagy | Autophagic Dysfunction Assay: Qualitative Analysis of MAP LC3I to LC3-II Conversion by Western Blot | U18 |
| Autophagic Dysfunction in LLC-PK1 Cells | U18 | |
| Immunology | Lymphocyte proliferation | U14 |
| Detection of Antibodies (anti PEG and others) | U2 | |
| Cell uptake | By cytometry | U14, U18 & 20 |
| By Confocal Microscopy | U2 | |
| Of metallic NPs/Fe-staining | U20 | |
| Optical Analysis: HR Dark-Field optical microscope with spectral analysis(Nano-scale Optical Microscope) | U12 |
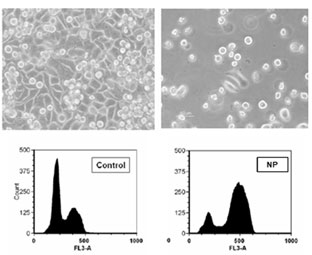
Effect of NP son cell viability and distribution in the cell cycle.
Elisa plate.
Phalloidin and DAPI staining of human pigmented epithelial cells (ARPE cells).