Animal Facility-IVIS Spectrum equipment for bioluminiscence and
fluorescence recording in whole-body mice
Scientific leaders of the project: Francisco Miguel Sánchez-Margallo, Verónica Crisóstomo, Beatriz Moreno, Simó Schwartz, José Luis Pedraz, Salvador Gil &Ramón Mangues.
Coordinator of the project: Ibane Abasolo & Francisco Miguel Sánchez-Margallo.
Industrial problem/gap covered
One of the causes that increase the time to market of nanotherapeutics is its preclinical validation. The standard protocols for more traditional drugs are not applicable for nanomedicines. NANBIOSIS offers a preclinical study plan tailored to meet the demands of each nanomedicine product, developed in collaboration with the user and starting from a set of basic analytical tests to more sophisticated experiments under either GLP and non-GLP conditions.
Description
Studies of nanomedicines are conducted in animals utilizing a variety of models and state of the arte imaging techniques (fluorescence, bioluminescence, TAC, MRI, CT, Echography, etc,) and a wide variety of analysis, including clinal tests: hematology, biochemistry, coagulation, urinalysis, tumor markers, hormones, fertility studies, cardiac markers, metabolic study. We collaborate in the selection of the most suitable animal species and can developexperimental pathology in different animal models (large white pig, sheep, goat, beagle dog, minipig, rabbit, rat and mouse) upon request.
Some examples are described in the following publications
Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide. Ferrer-Font L, Arias-Ramos N, Lope-Piedrafita S, Julià-Sapé M, Pumarola M,Arús C, Candiota AP. NMR Biomed. 2017Sep;30(9). doi: 10.1002/nbm.3748. Epub 2017 Jun 1. PubMed PMID: 28570014.
Pharmacokinetics of Benznidazole in Healthy Volunteers and Implications in Future Clinical Trials.Molina I, Salvador F, Sánchez-Montalvá A, Artaza MA, Moreno R, Perin L, Esquisabel A, Pinto L, Pedraz JL. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01912-16. doi: 10.1128/AAC.01912-16. Print 2017 Apr.
Repeated dose toxicity study of Vibrio cholerae-loaded gastro-resistant microparticles.López Y, Pastor M, Infante JF, Díaz D, Oliva R, Fernández S, Cedré B, Hernández T, Campos L, Esquisabel A, Pedraz JL, Perez V, Talavera A. J Microencapsul. 2014;31(1):86-92. doi: 10.3109/02652048.2013.808278. Epub 2013 Jun 24.
Common swine models of cardiovascular disease for research and training. Crisóstomo V, Sun F, Maynar M, Báez-Díaz C, Blanco V, Garcia-Lindo M, Usón-Gargallo J, Sánchez-Margallo FM. Lab Anim (NY). 2016 Feb;45(2):67-74. doi: 10.1038/laban.935.
Delayed administration of allogeneic cardiac stem cell therapy for acute myocardial infarction could ameliorate adverse remodeling: experimental study in swine. Crisostomo V, Baez-Diaz C, Maestre J, Garcia-Lindo M, Sun F, Casado JG, Blazquez R, Abad JL, Palacios I, Rodriguez-Borlado L, Sanchez-Margallo FM. J Transl Med. 2015 May 12;13:156. doi: 10.1186/s12967-015-0512-2.
In the development of this biomedical solution, the following services are involved:
Efficacy
Efficacy studies of nanomaterial are conducted in animal utilizing a variety of models. We collaborate in the selection of the most suitable animal species and can developexperimental pathology in different animal models (large white pig, sheep, goat, beagle dog, minipig, rabbit, rat and mouse) upon request. At present we have some models of pathologies available:
|
Patology |
Animal Model |
Unit |
References |
| Skin |
Wounds/skin regeneration |
Rat |
U10 |
|
| Skin ulcers |
Diabeticpig, mouse |
U19, 21, 22 & 24 |
|
| Diabeticulcers in cornea |
Rabitt |
U19, 21, 22 & 24 |
|
| Any ulcer model |
Pig, mouse |
U19, 21, 22 & 24 |
|
| Eye |
Ocular pathologies |
Rabbit |
U19, 21, 22 & 24 |
|
| Diabetes |
Diabetes (Type II) |
Pig |
U19, 21, 22 & 24 |
|
| Skin ulcers |
Diabetic pig, Rabit |
U19, 21, 22 & 24 |
|
| Diabetic ulcers in cornea |
Rabitt |
U19, 21, 22 & 24 |
|
| Bone |
Bone regeneration: calotte & hip |
Rat |
U10 |
|
| Brain |
Alzheimer |
Mouse |
U10 |
|
| Parkinson |
Rat |
U10 |
|
| Cerebral ischemia |
Rat |
U19, 21, 22 & 24 |
|
| Heart/Vascular |
Myocardial Infarction |
Pig |
U19, 21, 22 & 24 |
Crisostomo et al 2015 and 2017 |
| Vascular stenosis |
Pig |
U19, 21, 22 & 24 |
|
| Arterial embolization |
Pig |
U19, 21, 22 & 24 |
|
| Aneurysms |
Pig |
U19, 21, 22 & 24 |
|
| Cancer |
Colorectal* |
Immunodeficient mouse. Orthotopic, subcucaneous, intrasplenic. |
U18 & U20 |
Botella et al., 2011; Fernández et al., 2015 |
| Colorectal |
Immunocompetent (ApcMin) |
|
Mateo-Lozano et al., 2017 |
| Pancreas* |
Immuno deficient mouse. Subcutaneous. |
U18 & U20 |
Abasolo et al., 2009; Pujal et al., 2009 |
| Endometrium |
Immuno deficient mouse. |
U18 |
|
| Glioblastoma* |
Immunodeficient mouse. Orthotopic (steroatactic), subcucaneous. |
U18 & U20 |
Candiota et al., 2014 |
| Lymphoma* |
Immuno deficient mouse. |
U18 |
|
| Leukemia* |
Immuno deficient mouse. |
U18 |
|
| Prostate* |
Immuno deficient mouse. Orthotopic, subcucaneous. |
U20 |
Mateo et al., 2014 |
| Patient-derived tumor graft (PDX) models (Endometrium, colorectal) |
Immuno deficient mouse. |
U18 |
|
| Breast (breast & Breast ductal carcinoma, adenocarcinoma & ductal adenocarcinoma,)* |
Orthotopic (Intramamary fat pad). |
U20 |
Gener et al., 2015 |
| Cervix |
Subcutaneous. |
U20 |
Edinger et al., 1999 |
| Lung |
Subcutaneous. |
U20 |
Jakubowska et al., 2013 |
| Ewing sarcoma* |
Subcutaneous. |
U20 |
Ramon et al., 2013 |
| Skin: Melanoma* |
Subcutaneous & intravenous. |
U20 |
Adiseshaiah et al., 2014 |
| Glioblastoma |
Immuno competent mouse |
U25 |
Ferrer-Font L, et al. 2017 |
| Pseudotumors |
Pig |
U19, 21, 22 & 24 |
|
| Inflammation |
Inflammation |
Rat and mice (by LPS) |
U20 |
Thomas, Bath, Stover, Lambert, & Thompson, 2014 |
| Digestive |
Hernias |
Pig |
U19, 21, 22 & 24 |
|
| Colecystitis |
Pig |
U19, 21, 22 & 24 |
|
| Genitourinary |
Urethral obstruction and stenosis/ endothelium inflammation |
Pig |
U19, 21, 22 & 24 |
|
| Unilateral and bilateral cryptorchidism |
Dog |
U19, 21, 22 & 24 |
|
| Benign prostatic hiperplasia |
Dog |
U19, 21, 22 & 24 |
|
| Endometryosis |
Sheep |
U19, 21, 22 & 24 |
|
| Lysosomal storage disorders |
Fabry disease |
Immunocompetent mouse, KO for GLA gene |
U20 |
Cabrera et al., 2016
|
*Marked (luciferase, GFP, others)
REFERENCES
Abasolo, I., Pujal, J., Rabanal, R. M., Serafin, A., Navarro, P., Millán, O., & Real, F. X. (2009). FDG PET imaging of Ela1-myc mice reveals major biological differences between pancreatic acinar and ductal tumours. European Journal of Nuclear Medicine and Molecular Imaging, 36(7), 1156–1166.
Adiseshaiah, P. P., Patel, N. L., Ileva, L. V, Kalen, J. D., Haines, D. C., & McNeil, S. E. (2014). Longitudinal imaging of cancer cell metastases in two preclinical models: a correlation of noninvasive imaging to histopathology. Int J Mol Imaging. https://doi.org/10.1155/2014/102702
Botella, P., Abasolo, I., Fernández, Y., Muniesa, C., Miranda, S., Quesada, M., … Corma, A. (2011). Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation. Journal of Controlled Release, 156(2), 246–257. https://doi.org/10.1016/j.jconrel.2011.06.039
Cabrera, I., Abasolo, I., Corchero, J. L., Elizondo, E., Gil, P. R., Moreno, E., … Veciana, J. (2016). α-Galactosidase-A Loaded-Nanoliposomes with Enhanced Enzymatic Activity and Intracellular Penetration. Advanced Healthcare Materials, 5(7), 829–840. https://doi.org/10.1002/adhm.201500746
Crisóstomo V, Sun F, Maynar M, Báez-Díaz C, Blanco V, Garcia-Lindo M, Usón-Gargallo J, Sánchez-Margallo FM.Common swine models of cardiovascular disease for research and training. Lab Anim (NY). 2016 Feb;45(2):67-74. doi: 10.1038/laban.935.
Crisostomo V, Baez-Diaz C, Maestre J, Garcia-Lindo M, Sun F, Casado JG, Blazquez R, Abad JL, Palacios I, Rodriguez-Borlado L, Sanchez-MargalloDelayed administration of allogeneic cardiac stem cell therapy for acute myocardial infarction could ameliorate adverse remodeling: experimental study in swine. FM. J TranslMed. 2015 May 12;13:156. doi: 10.1186/s12967-015-0512-2.
Edinger, M., Sweeney, T. J., Tucker, A. A., Olomu, A. B., Negrin, R. S., & Contag, C. H. (1999). Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. https://doi.org/http://dx.doi.org/10.1016/S0959-8049(02)00410-0
Ferrer-Font L, Arias-Ramos N, Lope-Piedrafita S, Julià-Sapé M, PumarolaM,Arús C, Candiota AP. Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide. NMR Biomed. 2017Sep;30(9). doi: 10.1002/nbm.3748. Epub 2017 Jun 1. PubMed PMID: 28570014.
Fernández, Y., Forarada, L., García-Aranda, N., Mancilla, S., Suárez-López, L., Céspedes, M. V., … Abasolo, I. (2015). Bioluminescent Imaging of Animal Models for Human Colorectal Cancer Tumor Growth and Metastatic Dissemination to Clinically Significant Sites. Journal of Molecular Biology and Molecular Imaging, 2(2), 1019–33.
Gener, P., Gouveia, L. P., Sabat, G. R., de Sousa Rafael, D. F., Fort, N. B., Arranja, A., … Schwartz, S. (2015). Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine: Nanotechnology, Biology, and Medicine, 11(8), 1883–1892. https://doi.org/10.1016/j.nano.2015.07.009
Jakubowska, M., Śniegocka, M., Podgórska, E., Michalczyk-Wetula, D., Urbanska, K., Susz, A., … Plonka, P. M. (2013). Pulmonary metastases of the A549-derived lung adenocarcinoma tumors growing in nude mice. A multiple case study. Acta Biochimica Polonica. https://doi.org/10.1017/CBO9781107415324.004
Mateo-Lozano, S., Bazzocco, S., Rodrigues, P., Mazzolini, R., Andretta, E., Dopeso, H., … Arango, D. (2017). Loss of the EPH receptor B6 contributes to colorectal cancer metastasis. Scientific Reports, 7, 43702.
https://doi.org/10.1038/srep43702
Mateo, F., Meca-Cortés, Ó., Celià-Terrassa, T., Fernández, Y., Abasolo, I., Sánchez-Cid, L., … Thomson, T. M. (2014). SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Molecular Cancer, 13(1), 237. https://doi.org/10.1186/1476-4598-13-237
Pujal, J., Huch, M., José, A., Abasolo, I., Rodolosse, A., Duch, A., … Real, F. X. (2009). Keratin 7 promoter selectively targets transgene expression to normal and neoplastic pancreatic ductal cells in vitro and in vivo. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 23(5), 1366–1375.
Ramon, A. L., Bertrand, J. R., De Martimprey, H., Bernard, G., Ponchel, G., Malvy, C., & Vauthier, C. (2013). SiRNA associated with immunonanoparticles directed against cd99 antigen improves gene expression inhibition in vivo in Ewing’s sarcoma. Journal of Molecular Recognition. https://doi.org/10.1002/jmr.2276
Thomas, R. C., Bath, M. F., Stover, C. M., Lambert, D. G., & Thompson, J. P. (2014). Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides. https://doi.org/10.1016/j.peptides.2014.08.009
Pharmacology and biodistribution
- Pharmacokinetic; Animal studies in small and big animals to determine ADME (absorption, distribution, metabolism and excretion) studies.
- Quantification of nanomedicines in fluids and biomarkers by ELISA, fluorescence and analytical techniques
- Validation and optimization of techniques
- Biodistribution by MRI, luminescence, CT or echography
- Studies in tissues by coupling hyperpolarization and MRI/MRS/MRSI at 7Tesla
- Metabolomics studies in biofluid by NMR (14T)
Molina I, Salvador F, Sánchez-Montalvá A, Artaza MA, Moreno R, Perin L, Esquisabel A, Pinto L, Pedraz JL. Pharmacokinetics of Benznidazole in Healthy Volunteers and Implications in Future Clinical Trials.Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01912-16. doi: 10.1128/AAC.01912-16. Print 2017 Apr.
Toxicity and Immunotoxicity
| Single-Dose Acute Toxicity and Repeat-Dose Acute Toxicity Studies of anticancer nanomedicines in small animals |
U18 & U20 |
Rafael et al., 2017 |
| Histopathology in small animals |
U18 |
Candiota et al., 2014 |
| Single-Dose Acute Toxicity and Repeat-Dose Acute Toxicity Studies of nanomedicines in large animals |
U19, 21, 22 & 24 |
|
| Histopathology in large animals |
U19, 21, 22 & 24 |
|
| Detection immune response: detection of Antibodies (anti PEG and others) |
U2 |
|
| PK of therapeutic antibodies |
U2 |
|
References
Candiota, A., Acosta, M., Simões, R., Delgado-Goñi, T., Lope-Piedrafita, S., Irure, A., … Arús, C. (2014). A new ex vivo method to evaluate the performance of candidate MRI contrast agents: a proof-of-concept study. Journal of Nanobiotechnology, 12(1), 12. https://doi.org/10.1186/1477-3155-12-12
Rafael, D., Martínez, F., Andrade, F., Seras-Franzoso, J., Garcia-Aranda, N., Gener, P., … Schwartz, S. (2017). Efficient EFGR mediated siRNA delivery to breast cancer cells by Cetuximab functionalized Pluronic® F127/Gelatin. Chemical Engineering Journal. https://doi.org/10.1016/J.CEJ.2017.12.114
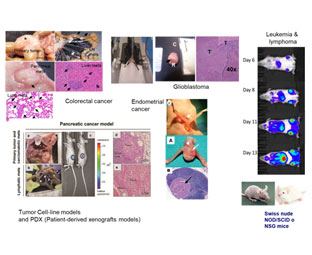
Mouse models for experimental oncology.
Nasal cavity.
1.5T MRI.



